Retinoic Acid Signaling Involved in Endometrial Decidualization
Jun 27, 2017
Unsuccessful IVF in women with endometriosis may be linked to altered Retinoic Acid Signaling.
Key Points
Highlights:
- Researchers from the Northwestern University School of Medicine have observed significant differences in the uptake, metabolism, and action of retinoic acid in the cells of endometriosis tissue, compared to eutopic endometrium in preparation for pregnancy - a process called decidualization.
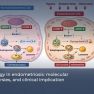
- The genes involved in retinoid uptake and action (such as CRABP1 and CRABP2) are decreased, while those participating in the elimination of retinoid acid were increased, compared to normal endometrium. The retinoic acid cascade genes and their ratio are necessary for the appropriate decidualization. Furthermore, the lack of another paracrine molecule, RBP4, in endometriosis during decidualization, may be the cause of increased estradiol levels.
Importance:
- Endometriosis is an estrogen-dependent disorder and is often associated with subfertility through the effects on oocyte development, embryogenesis or implantation. Many IVF clinics have observed that women with endometriosis have decreased success rates of implantation.
- Previous studies revealed reduced implantation, as well as altered receptivity in the endometrium, may be caused by its reduced capacity to decidualize.
- Also, it is known that retinoid signaling pathways are altered in endometriosis. Hence, alterations in the retinoid pathway in endometriotic tissue may be a contributing factor to the lower implantation rates seen in patients with endometriosis who undergo IVF.
Key Results:
- The genes involved in retinoid uptake and action (such as CRABP1 and CRABP2) are decreased in the stromal cells of endometriosis, while those participating in the elimination of retinoid acid are increased, compared to normal endometrium. In other words, impaired endometrium in the decidualization of endometriotic cells may be mediated by genes in the retinoic acid signaling cascade.
- When CRABP2 was eliminated from the normal endometrial cells, decidualization is disturbed, conversely, when FABP5 has removed from endometriosis stromal cells, their ability to decidualize is increased indicating CRABP2 and FABP5 have a significant antagonistic role in the decidualization process and their ratio is critical for the cell fate regulating the appropriate decidualization.
- Another paracrine molecule, RBP4, is present in endometrium but not in endometriosis cells typically induces the enzyme 17-beta-dOH-type 2 (HSD17B2) to inactivate excess estradiol during decidualization. The absence of RBP4 in endometriosis may be the reason for the excess levels of estradiol ensuing in a vicious cycle.
What’s done here?
- 17 samples of eutopic endometrium from hysterectomies and 92 endometriosis tissues were obtained from ovarian endometrioma cyst walls; grown in culture and induced for in vitro decidualization.
- Molecules involved in the retinoic acid pathway, such as cellular retinoic acid binding protein 1,2 (CRABP1 and 2), FABP5, prolactin, estradiol, and others were analyzed.
Limitations:
- The studies were performed in endometriosis tissue, not in eutopic endometrium of endometriosis patients. Eutopic endometrium came from independent patients.
Lay Summary
Decidualization occurs in the eutopic endometrium during the luteal phase and is controlled by signaling pathways such as cyclic adenosine monophosphate (cAMP) and progesterone. Researchers from the Northwestern University School of Medicine reported in their recent paper entitled "Altered Retinoid Signaling Compromises Decidualization in Endometriotic Stromal Cells" which appeared in the scientific journal Reproduction, that they found significant differences in the uptake, metabolism, and action of the retinoic acid pathway.
Many IVF clinics have observed that women with endometriosis have reduced success rates in part due to decreased decidualization capabilities of the eutopic endometrium of endometriosis patients. Thus, alterations in the retinoic acid pathway in endometriotic tissue may be a contributing factor to the lower implantation rates seen in patients with endometriosis who undergo IVF.
Normal endometrial tissue obtained from hysterectomies from 17 patients and endometriotic tissue from ovarian endometriomas from 15 patients. Normal endometrial and endometriotic cell were subjected to in vitro culture and then decidualization (IVD) in the laboratory and then analyzed for molecules involved in the retinoic acid pathway, such as cellular retinoic acid binding protein 1,2 (CRABP1 and CRABP2), prolactin, and others.
Stromal cells of endometriosis showed a decrease in the genes involved in retinoid uptake and action (such as CRABP1 and CRABP2) while those participating in the elimination of retinoid acid were increased, compared to normal endometrium stroma. When CRABP2 was eliminated from the normal endometrial cells, prolactin levels were significantly decreased- indicating that CRABP2 has a role in the ability for normal endometrium to decidualize and function properly. Conversely, when FABP5 was eliminated from endometriotic stromal cells, prolactin levels increased, and their ability to decidualize is increased. Thus, CRUBP2 and FABP5 ratio may play antagonistic roles on the endometrium, and their ratio is critical for the cell fate in preparation for pregnancy. Researchers also found that another paracrine molecule, RBP4, is secreted in the endometrium, but not in endometriosis cells during decidualization which functions to induce the enzyme 17-beta-dOH-type 2 (HSD17B2) is also important because HSD17B2 is absent in endometriosis thus estradiol can not be inactivated.
These findings could be used to understand the mechanism of implantation and applied for the management of disturbed situations such as endometriosis for the women to experience a successful pregnancy.
Research Source: https://www.ncbi.nlm.nih.gov/pubmed/28592664
Retinoic acid signaling decidualization menstrual cycle IVF endometriosis. HSD17B2 CRABP1 CRABP2 RBP4 FABP5. implantation oocyte embryogenesis